2.1 Combustion phenomenon
When heated from the bottom, the typical combustion process of lithium-ion battery packs can be roughly divided into the following six stages based on the severity of combustion.
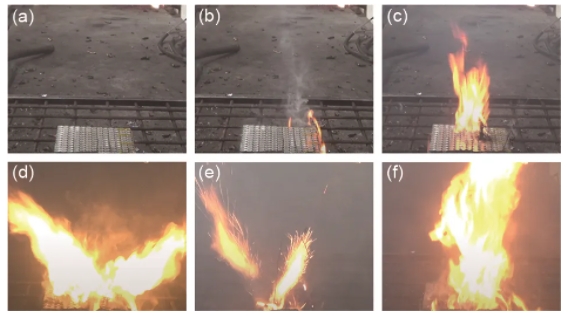
(1) Heating stage. After the heating furnace continuously heats the negative electrode of the lithium battery pack for a period of time, smoke will gradually form on the surface of the battery, some of which comes from the damage of the positive electrode of the lithium battery cell. This is because the positive electrode cap of the battery cell can be seen being washed away by air pressure at the experimental site or in the video. This indicates that a violent reaction occurred inside the battery due to continuous heating, producing a large amount of gas, followed by a small amount of electrolyte emerging from the positive electrode of the battery, as shown in (a).
(2) Fire stage. In the experiment, it was observed that the battery produces a large amount of smoke before ignition, which contains many combustibles, including those generated from the internal reaction of the battery, as well as those generated from the thermal decomposition of the packaging material of the battery pack. After the heat accumulates to a certain extent, ignition occurs, as shown in (b).
(3) The ignition stage. For the experimental condition of heating from the bottom, the middle part of the lithium battery receives the most concentrated heat, as shown in the red dashed box in Figure 4 (a). Therefore, the middle part of the battery pack begins to be ignited on a large area, and then spreads to the surrounding areas. The material inside the battery cell is also sprayed out, as shown in (c).
(4) Spray stage. After the surface of the lithium battery pack catches fire, the temperature rises sharply, causing the safety valves of the battery cells to be opened one by one, and then combustible gas is sprayed outward, as shown in (d), accompanied by the splashing of electrolyte everywhere, as shown in (e). At this stage, an obvious phenomenon is intense combustion and violent explosion sounds. The battery cells are also splashing everywhere under internal pressure, which makes it difficult to collect temperature changes during the test. The thermal couple arranged before the test are easily damaged or moved by this explosion, and cannot guarantee real-time measurement of temperature changes at the same location.
(5) The overall combustion stage. When the safety valves of most lithium battery cells are flushed open, the lithium battery pack enters the overall combustion stage, where the flame height is higher and the flame volume is larger, as shown in (f).
(6) Attenuation stage. After the combustible materials inside the battery are gradually consumed, the fire goes out.
When the lithium battery pack is heated from the side, the combustion process is shown in the figure, and the entire combustion stage is roughly similar to the bottom heating condition, including ignition, ignition, injection, and other processes. From the perspective of ignition time, except for a significant reduction in ignition time in Test 2, the two heating methods in this article do not have a significant impact on ignition time. However, after doubling the power, from 1 kW to 2 kW, for example, compared to Test 3 and Test 1, and Test 5 and Test 4, under the same heating position, an increase in power will significantly shorten ignition time.

As shown in (a), the side of the lithium battery pack is heated by an external radiation heat source. After a period of time, the battery pack closest to the heat source catches fire first, as shown in (b), and then burns and spreads from right to left.
The safety valve of the battery cell near the high-temperature flame is pushed open, producing white smoke, as shown in (c), accompanied by the phenomenon of sparks splashing, as shown in (d), This indicates that the electrolyte inside the battery cell is sprayed out. The flame on the surface of the battery pack gradually extinguishes during the propagation process, while the non ignited battery is still undergoing intense reactions inside.
At this time, a large amount of white smoke can be observed, as shown in (f), which is the gas produced by the internal reaction of the battery cell. When encountering high temperatures, sparks, or similar ignition energy, the battery undergoes re ignition, as shown in (g), until the flame is completely extinguished. From the test results, it can be seen that when the side of the lithium battery pack overheats, The intensity of battery combustion will gradually decrease with increasing distance, and intermittent reignite will occur multiple times.
Lithium battery cells are connected in series and parallel to form a battery pack during use, and each battery cell is intelligently managed through a Battery Management System (BMS). After being ignited by an external radiation heat source, the battery pack will undergo explosive and intense combustion. Some battery cells will detach from the battery pack under the pressure generated by internal reactions, splatter everywhere, and the internal diaphragm material will also overflow.
Due to the design of the positive electrode safety valve of the battery cell, when gas is generated by internal chemical reactions, pressure will leak out through the safety valve, thereby reducing the risk of damage to the outer wall of the battery cell caused by internal pressure.
For a lithium battery pack, if one of the batteries experiences thermal runaway ignition or catches fire due to external factors, the surrounding lithium battery cells will be subjected to heat transfer from the wall, or the high-temperature residue sprayed out will burn, or the thermal radiation generated by an open flame will ignite the surrounding battery cells, further expanding the ignition area without instantly expanding the fire due to wall explosion. This indicates that compared to the side of a lithium battery pack, Its bottom (negative electrode) needs to be better protected against fire.
The combustion spread process of lithium battery packs under overheated conditions first changes with the battery temperature. By measuring the changes and distribution of internal temperature in the battery pack, the fire behavior of lithium battery packs can be quantitatively analyzed. This is of great significance for how to better control the fire and develop new and efficient fire extinguishing technologies.
2.2 Temperature distribution
The change in internal temperature of a lithium battery pack when heated at the bottom. From the changes in the temperature history curve, three different stages of battery thermal runaway evolution can be observed, which is consistent with the results obtained from the analysis of combustion phenomena in the previous section.
During the heating stage, the temperature slowly rises, and it can be seen that the temperature of T 1 rises faster than others. Its measured position is just in the center area of the battery pack, where it receives the most heat. After the positive safety valve of the battery cell is opened, white smoke emerges, indicating that a violent reaction is occurring inside the battery cell, producing gas.
As the temperature continues to rise, the combustible gas produced triggers the ignition of the battery cell. At this point, the temperature curve shows a sharp upward turning point. At the time of ignition, the temperature of T 1 is about 139 ℃, and at the same time, the temperature at T 3 position immediately rises, which is caused by the spread of flames. Compared to positions T 2 and T 3, the straight-line distance between T 4 and T 1 is the farthest. If calculated based on the maximum size of the battery cell, the interval between T 4 and T 1 is about 99 mm, the ignition time difference is 89 s, and the combustion spread speed is about 1.1 mm/s.
After a lithium battery pack catches fire, it will experience phenomena such as spraying and explosion, during which the temperature will continue to rise and eventually enter the overall combustion stage. The maximum combustion temperature of the battery pack exceeds 700 ℃.
When the side of the lithium battery pack is heated, the measurement position of the thermal couple has been adjusted, arranged from near to far relative to the radiation heat source position, and the typical temperature history curve measured in the experiment has been adjusted.
Compared to the bottom heating condition, there are some significant differences in temperature distribution when the side of the lithium battery pack is heated. Although there are several peaks in temperature, which means that it is in an open flame combustion state at this time, the temperature peak distribution at the bottom heating is over a longer period of time. This is mainly because the heating conditions of the lithium battery pack are different under the two different working conditions.
When the bottom is heated, the negative electrode of the lithium battery cell is continuously heated, and most directly heated battery cells will accelerate chemical reactions within a more concentrated time period. After losing control of the heat, they will catch fire and eventually exhibit a phenomenon of overall combustion. However, when heated on the side, the position further away from the heat source, such as T 4, will receive less heat, and the temperature will not increase significantly until the end of combustion, This is mainly because when the battery pack is heated on the side, it exhibits a combustion spread pattern from right to left, and there will be extinguishing and reignite phenomena in the middle. Without continuous external heating, this combustion form is difficult to achieve continuous combustion spread.
In addition, it can be observed that the ignition temperature at T 1 during ignition is about 90 ℃, which is lower than when the bottom negative electrode is heated, and the highest temperature after ignition is also around 550 ℃, which is significantly lower than when the bottom is heated. The results of the above temperature analysis indicate that compared to side heating, the negative electrode on the bottom of the lithium battery pack experiences more severe combustion due to thermal runaway when heated, specifically manifested in a larger combustion spread area and higher flame temperature.
The temperature changes in working condition 3, compared to working condition 1, are all bottom heating, with the difference being that the heating power has increased to 2 kW. At the same time, in the middle of combustion, water mist is turned on to extinguish the fire and test its fire suppression and cooling ability. It can be observed that as the heating power increases, the ignition time is advanced. The reason is obvious. The higher the external heat received, the more intense the chemical reaction inside the battery cell will be, and the thermal runaway will lead to a shorter ignition time.
However, during ignition, there was no significant difference in temperature between T 1 and lower power operating conditions, but rather between 120 and 139 ℃. In addition, the temperatures at positions T 2, T 3, and T 4 in operating condition 3 are lower than those at a heating power of 1 kW. This may indicate that under the influence of external radiation heat, the lithium battery pack will only ignite and burn when the temperature rises to a specific range.
It should be pointed out that the ignition temperatures listed in Table 2 may not be the minimum ignition temperature obtained due to limited collection points. However, this can to some extent demonstrate the necessity of real-time monitoring of temperature rise changes for each battery cell through a battery management system. By setting a temperature threshold, once the local temperature rise of the lithium battery pack exceeds this value, warning and prevention measures will be initiated to prevent the entire battery pack from catching fire due to thermal runaway.
The whole duration of combustion is 218 s from the start of ignition at t=780 s to the start of water mist at t=998 s. After the water mist is turned on to extinguish the fire, the temperature at T 3 and T 4 drops rapidly. After continuous spray for 30 s, the temperature drops below 100 ℃. After the spray is stopped, no re combustion occurs. The experimental results show that water mist can effectively suppress fire and cool down, and prevent reignite.
Compared to gas fire extinguishing, water mist may be a good extinguishing medium that can continuously cool down lithium-ion batteries on fire. However, it should also be noted that in practical applications, water stains generated by water mist may cause extensive equipment damage and secondary damage. In addition, water mist with a large droplet diameter may also cause battery short circuits or discharge, exacerbating thermal runaway and increasing the scale of fires, Therefore, the specific selection of fire extinguishing methods needs to be analyzed based on the fire extinguishing object. And the basis for these specific applications is experimental data, so it is very necessary to further carry out larger scale, especially full-scale fire extinguishing experiments, to study the combustion characteristics of lithium battery packs and test the effectiveness of different fire extinguishing methods.
3 Conclusion
This article conducts combustion test on ternary 18650 lithium-ion battery packs induced by heating, and the following conclusions are obtained.
(1) Compared to overheating on the side, when the negative electrode on the bottom of a lithium battery pack overheats, the combustion degree is more severe, and the battery will continuously spray and burn. For side overheating, the intensity of combustion in lithium battery packs will weaken with increasing distance from the heat source, and multiple intermittent reignite phenomena will occur. In addition, an increase in heat source power will shorten the ignition time of lithium battery packs and increase their combustion intensity.
(2) The test results show that the thermal runaway temperature of the bottom negative electrode of the ternary lithium battery pack is between 120~139 ℃ when it overheats. Under these conditions, the maximum combustion temperature will increase with the increase of heat source power, and the maximum temperature can reach 800 ℃.
(3) Applying pure water mist to the burning lithium battery pack can effectively suppress the fire and reduce the temperature. Continuous spray can reduce the battery temperature below the critical temperature without reignite. This indicates that water mist can be an effective fire extinguishing method for lithium-ion battery fires, but its application may bring secondary damage such as water pollution and short circuit discharge, requiring careful selection based on fire extinguishing needs.
